
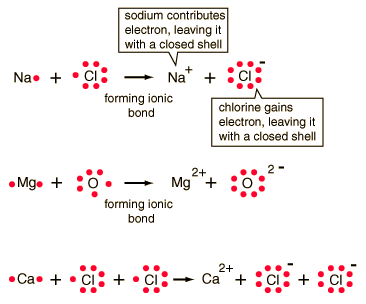
polymer “chains”), which themselves exhibit covalent bonding, typically between organic (i.e., carbon-containing) constituents. Polymers comprise a wide variety of materials that are characterized by the entanglement and interconnection of long molecules (a.k.a. It is this high bond strength and chemical inertness that make ceramics completely unaffected by UV exposure (see Endnote). In contrast to metals, ceramic ions have tightly bound electrons, hence they have a high bond strength, withstand extreme temperatures, are usually extremely chemically inert and are strong electrical insulators. Most ceramics are metal oxides, though some ceramics are nitrides, borides and carbides that exhibit strong covalent bonding. Suffice it to say that for nearly all applications, metals can be considered impervious to UV degradation.Ĭeramics are characterized primarily by ionic bonding, the attraction of positively and negatively charged ions arranged in a periodic lattice structure. These effects are hypothesized to be related to photoelectric effects between the surface oxide layer and the underlying metal. There is some published evidence 1 of increases in the corrosion rates of metals immersed in water exposed to UV, but the results are of questionable significance, and the metals that seem to be most susceptible are not good candidates for immersion anyway.
#DOES ION BONDING CHANGE THE COLOR OF STAINLESS FREE#
Metals are almost entirely unaffected by UV because of the availability of free electrons to absorb photon energy without undergoing energy transitions or bond dissociation. This explains why metals are never transparent and almost always reflect light to some degree.
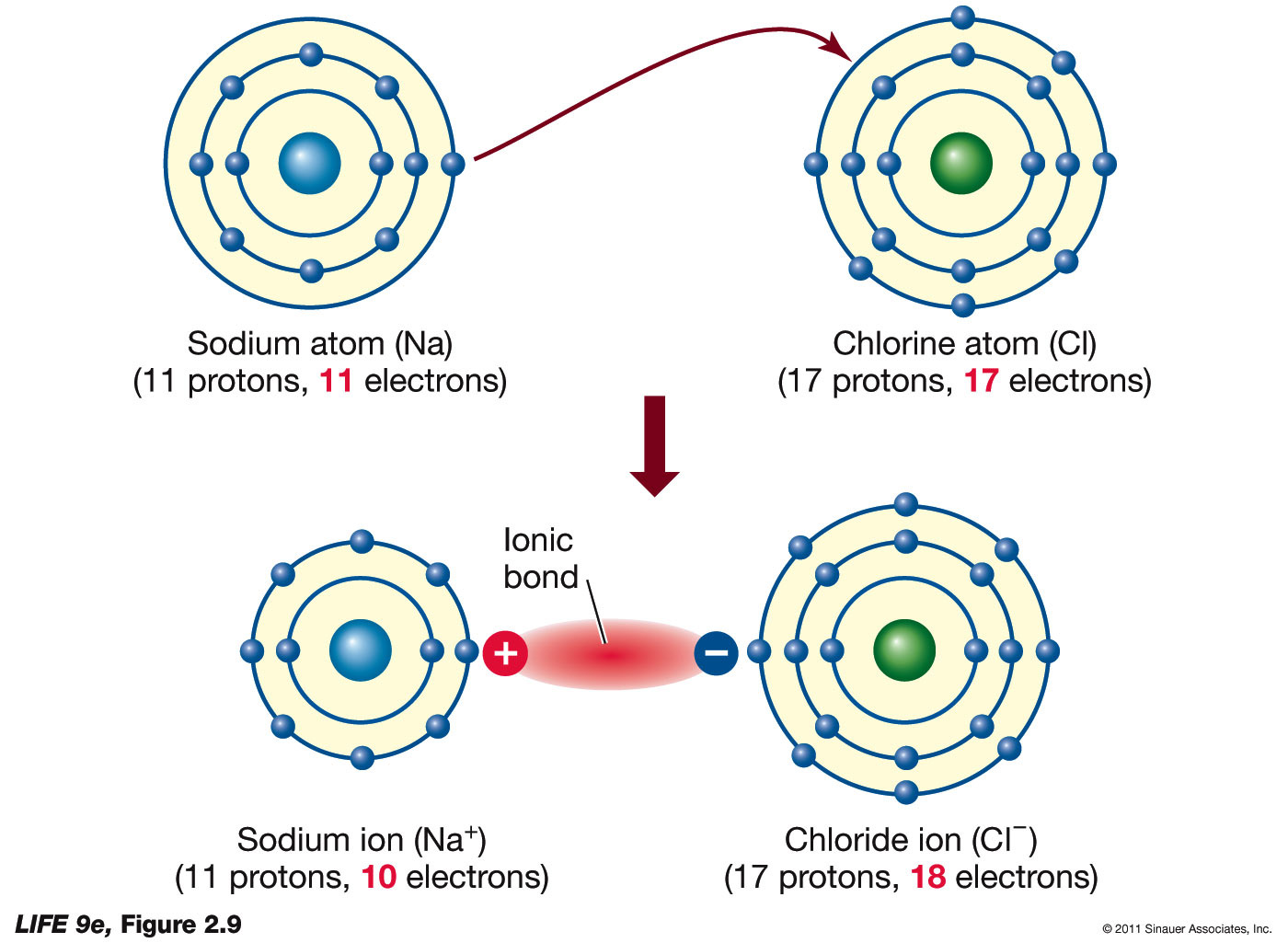
Because of their highly mobile electrons, metals are good conductors of electricity and heat, and readily interfere with electromagnetic radiation such as light and radio waves.
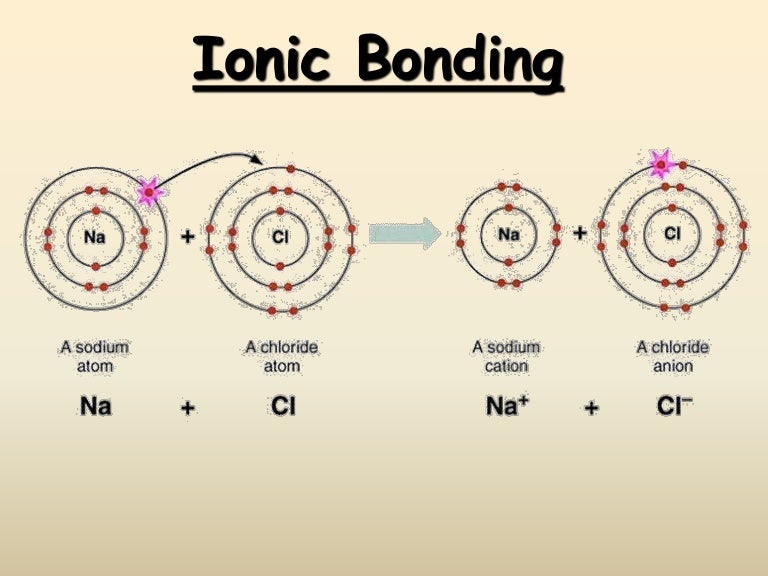
Metals are characterized by metallic bonding, which is defined by tightly packed atoms arranged in a periodic lattice structure all sharing a “cloud” of delocalized electrons. Such an overview must start with a basic lesson in materials science, explaining the three broad classes of materials as classified by their atomic bonding characteristics. The intent of this article is to give an overview of the broad classes of materials and an explanation of their susceptibility (or lack thereof) to UV-induced degradation. Knowledge of the effects of UV-C exposure on various materials is useful to manufacturers and users of UV-C disinfection and photochemical equipment. Granted, sunlight-induced damage may be a reasonable predictor for what could be expected to occur with UV-C exposure, but the high-energy UV-C photons can have unique effects that cannot always be predicted by solar UV exposure. As a crudely demonstrative example, a search in Google Scholar for “UV degradation” results in over three million hits, while “UV-C degradation” results in only around 19,000 hits. Therefore, published data on materials’ degradation by UV-C is minimal.

However, UV-C, the subclass of UV radiation with wavelengths between 200 and 280 nm, is not present in terrestrial sunlight because wavelengths lower than ~300 nm are absorbed by the ozone layer in the upper atmosphere. Accordingly, the published data on material degradation by UV is almost exclusively relevant to that present in sunlight at the earth’s surface, as this represents such a large economic impact. The degradation effects of UV are of concern to designers and users of a wide variety of materials that are intended for use and storage outdoors and thereby exposed to sunlight. Production and applications engineer, LightSources, Inc.Īs ultraviolet (UV) radiation consists of photons with high energy relative to visible light, it can cause degradation in the form of physical and chemical changes in susceptible materials.


 0 kommentar(er)
0 kommentar(er)
